5 Which Statement Best Describes Esters
Which statement best describes how irony is used in this excerpt. Fe Cu2 Fe2 Cu a.

Schematic Representation Of The Most Important Wine Esters Ethyl Download Scientific Diagram
Esters are organic acids that have a strong smell and a sour taste B.

. Esters are compounds that form when organic acids react with alcohols. Esters are also usually derived from carboxylic acids. C Maybe you like.
This ability to participate in the. Choose the one alternative that best completes the statement or answers the question. A Esters are hydrocarbons that contain ring structures.
Esters are derivative of carboxylic acids where the hydroxyl OH group has been replaced by an alkoxy O-R group. Which statement best describes esters. Esters have pleasant smells and are used in perfumes together with other compounds.
The structure is the product of a carboxylic acid the R-portion and an alcohol the R. Esters are used as flavourings and fragrances. Where the symbols R1 and R2 represent organic radicals.
Carboxylic acid esters formula RCOOR R and R are any organic combining groups are commonly. Which statement best describes esters. Esters are used as an organic solvent 3.
Answered expert verified Which statement best describes esters A. Esters that are have fragrant odours are used as a constituent of perfumes essential oils food flavourings cosmetics etc 2. Choose the statement which best describe ester.
In this reaction both iron and copper are reduced. Natural esters are found. RCO2HROH RCO2RH2O R C O 2.
In this reaction iron is oxidized and copper is reduced. In this reaction iron is reduced and copper is oxidized. To put it in simple terms esters are the group of chemical compounds which are formed by bonding of an alcohol group with a group of organic acids by losing water molecules.
Esters are organic chemical compounds whose structure has the general form. C Esters are compounds that form when organic acids react with alcohols. Any questions for which more than one response has been blackened.
It is used in paints and nail varnish remover. 9 hours agoQuestion and answer. This could be an alkyl group like methyl or ethyl or one containing a benzene ring like phenyl.
Ester any of a class of organic compounds that react with water to produce alcohols and organic or inorganic acids. Describe the conditions needed to produce esters. These chemical compounds participate in the formation of hydrogen bonds as the acceptors of hydrogen-bond but they cannot act as the hydrogen-bond donors.
A carboxylic acid contains the -COOH group and in an ester the hydrogen in this group is replaced by a hydrocarbon group of some kind. Esters are made from carboxylic acids and alcohols. 25 Multiple choice questions 5 free response questions for a total of 100 points and one extra credit question.
B Esters are organic acids that have a strong smell and a sour taste. They are commonly synthesized from the condensation of a carboxylic acid with an alcohol. The term ester was introduced in the first half of the 19th century by German chemist Leopold Gmelin.
They are volatile and so are easily separated from the solute. A Which statement best describes the following reaction. On treating with sodium hydroxide ester is converted back.
Ester are formed due to the reaction between an acid and a carboxylic acid. An ester is an organic compound that is a derivative of a carboxylic acid in which the hydrogen atom of the hydroxyl group has been replaced with an alkyl group. Name Date ANSWER ALL QUESTIONS.
Esters are derived from carboxylic acids. Ethyl ethanoate is a common solvent due to its low cost and low toxicity. Once a flower or fruit has been chemically analyzed flavor chemists can attempt to duplicate the natural odor or taste.
Esters derived from carboxylic acids are the most common. This lesson describes the uses and structure of esters. The mechanism of ester formation is described as well as biological applications such as saponification and.
Note Fats and vegetable oils are esters of long-chain fatty acids and glycerol. Esters are organic acids that have a strong smell and. Esters are used as solvents for organic compounds.
Ethanoic acid ethanol ethyl ethanoate water with sulphuric acid as a catalyst CH 3 COOH C 2 H 5 OH CH 3 COOC 2 H 5 H 2 O. Esters are hydrocarbons that contain ring structures Advertisement Expert-verified answer IlaMends AnswerThe correct answer is option B. Ester is formed due to the reaction between ethanol and Ethanoic acid.
Esters are sweet smelling substances. Esters are hydrocarbons that contain ring structures. The making of esters is also called esterification.
Math Chemistry Biology Programming Arts History BusinessLanguage Spanish EnglishTipsReviewBlog Home Which statement best describes esters April 27 2022 thanh Esters are hydrocarbons that contain ring structuresb Esters are organic acids that have. April 27 2022 thanh. R 1 and R 2 are not necessarily the same as each.
Both natural and synthetic esters are used in perfumes and as flavoring agents. Esters of phosphoric acid are of the utmost importance to life. B and C describe esters.
In this reaction both iron and copper are oxidized. A Modest Proposal I profess in the sincerity of my heart that I have not the least personal interest in endeavouring to promote this necessary work having no other motive than the public good of my country by advancing our trade providing for. R 1 and R 2 are often carbon chains that can be either linear or branched and might also have other functional groups attached.
Esters are polar in nature but not more than alcohols.
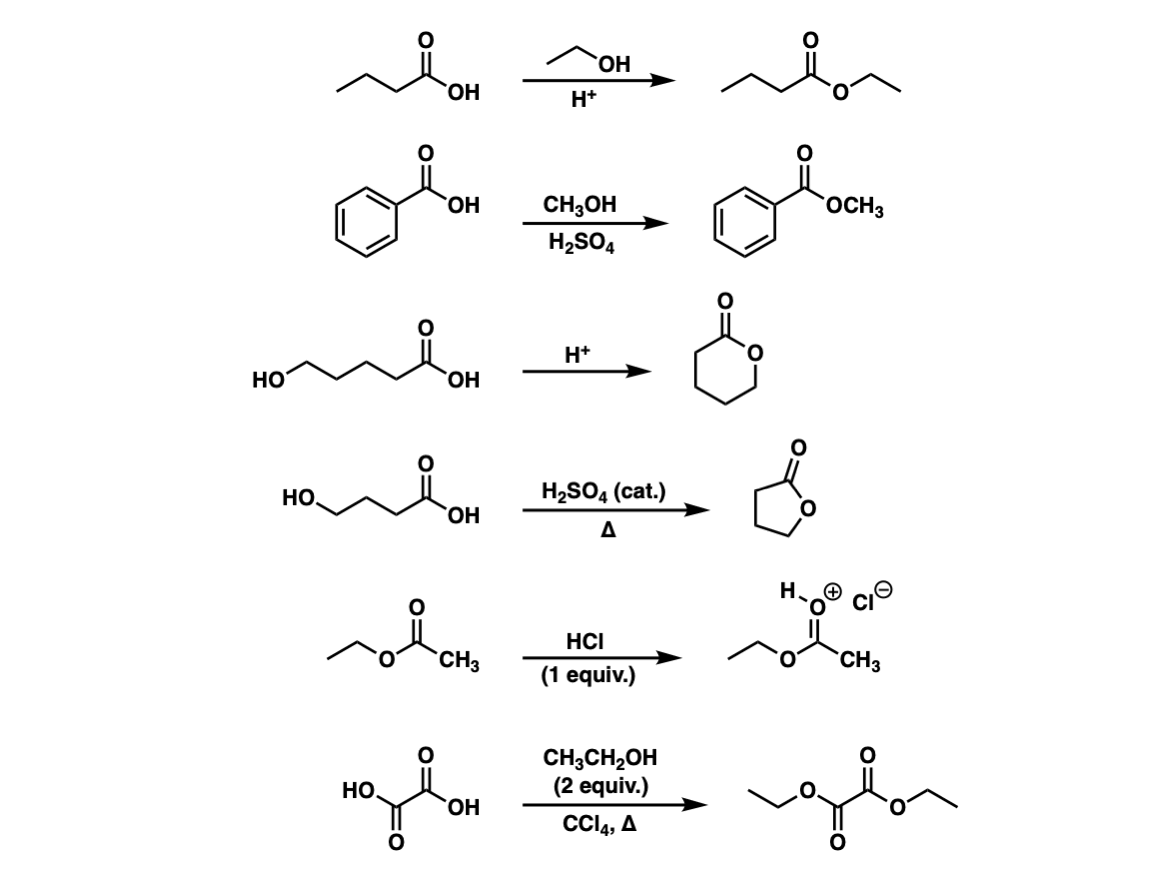
Conversion Of Carboxylic Acids To Esters Using Acid And Alcohols Fischer Esterification Master Organic Chemistry
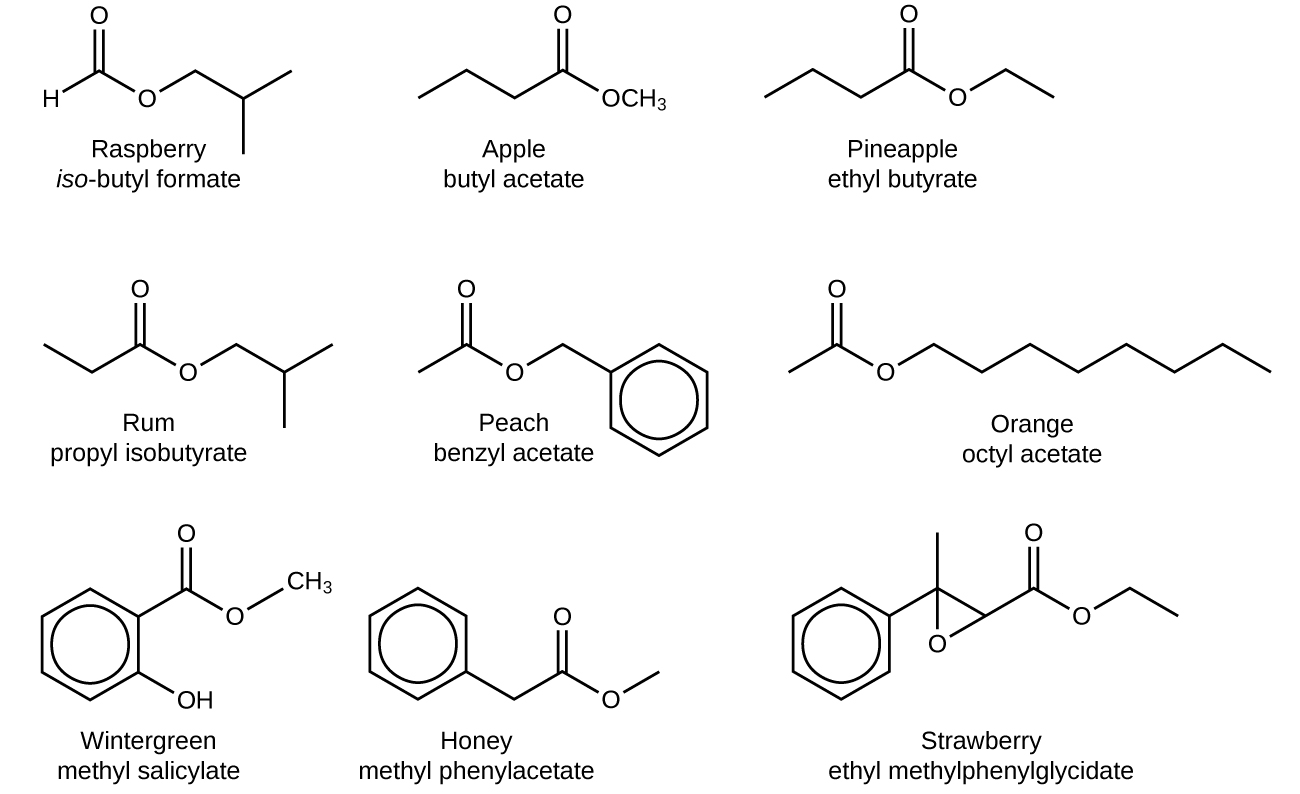
20 3 Aldehydes Ketones Carboxylic Acids And Esters Chemistry
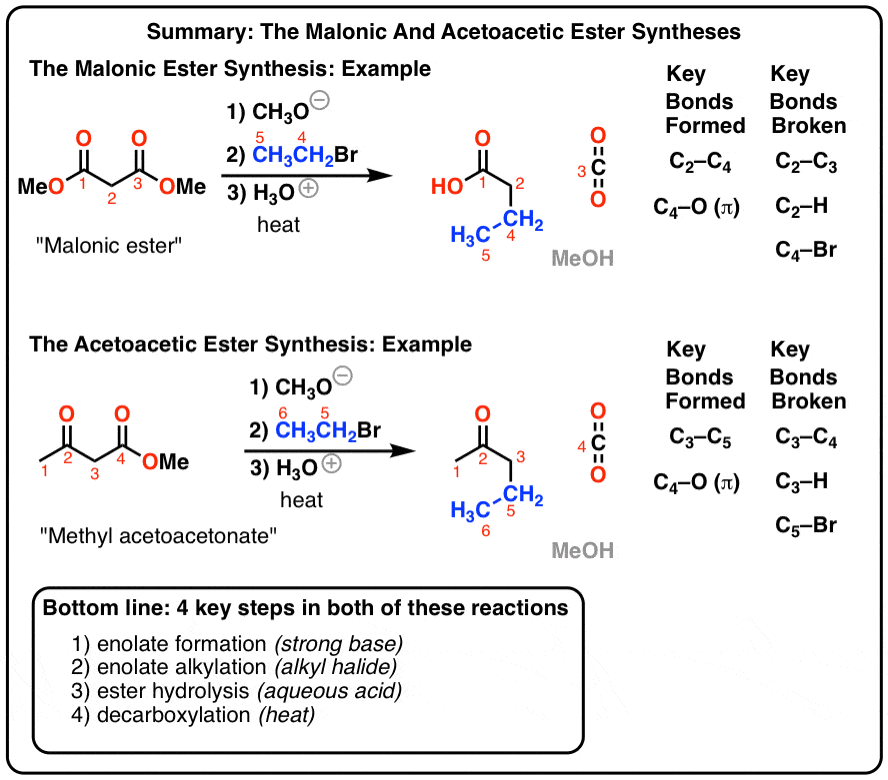
The Malonic Ester Synthesis Master Organic Chemistry

Disposal Of Controlled Substances Met Bio Medical Waste Management Drugs Abuse Medical
No comments for "5 Which Statement Best Describes Esters"
Post a Comment